
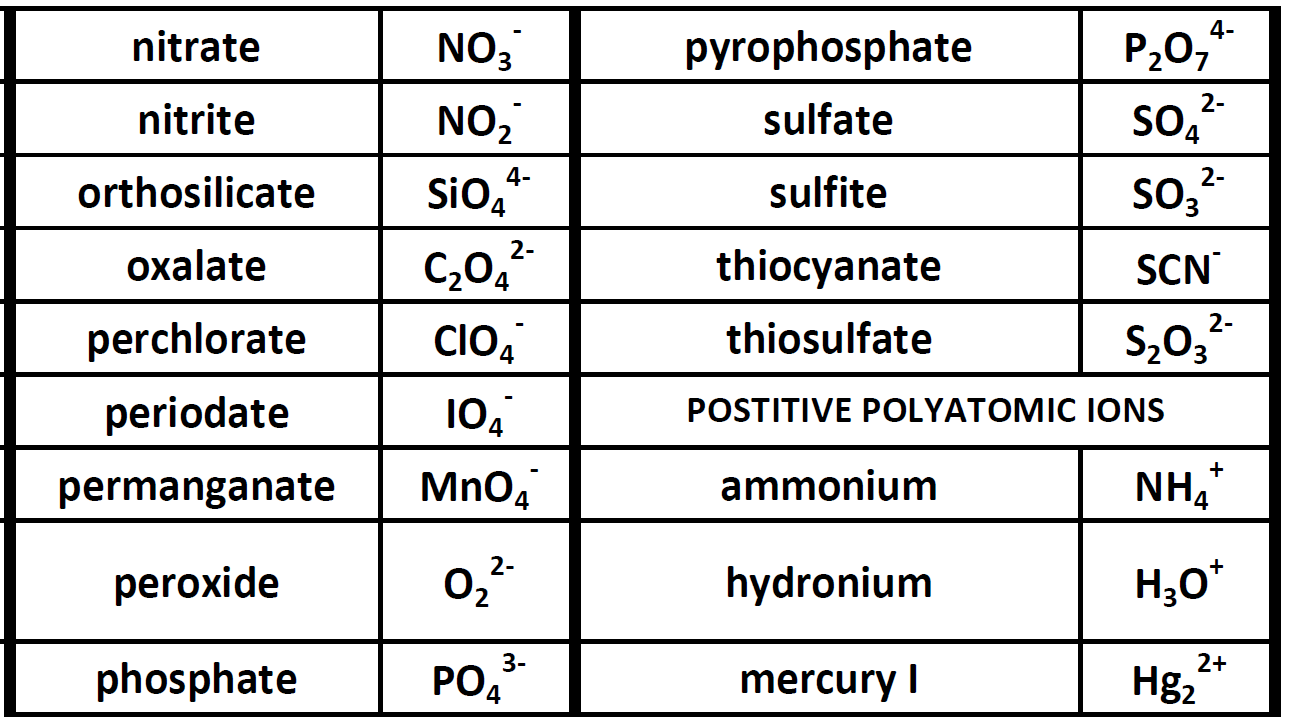
Identify the main group to which X belongs in each ionic compound formula: X2O3 XCO3 Na2X. The sign of the charges in the subscript is ignored while writing the molecular formula. In general, when does the lattice energy of an ionic compound increase in relation to the charges and sizes of the ions Charges on the ions: increase or decrease Sizes of the ions: increase or decrease 3A 2A 6A. However, one Ca 2+ cation requires 2 Cl atoms as 2Cl - to balance the charges and form a neutral compound.īy criss-crossing the numerical value of charges and using it as a subscript, the correct ratio of the ions is obtained to conclude the formula of AB 2 as CaCl 2. The valency of Cl is 1, and one Cl atom can accept one electron to form one Cl - anion. The valency of Ca is 1, and the one Ca atom comfortably loses two electrons to form Ca 2+ cation. The simplest method is to employ the criss-cross method. Criss-cross (cross-multiplication) method to deduce the Molecular Formula One Ca atom, and two Cl atoms form the compound Calcium Chloride (CaCl 2). Nonmetal atoms in polyatomic ions are joined by covalent bonds, but the ion as a whole participates in ionic bonding. Several examples are found in Table 3.3.1.

The atoms of a polyatomic ion are tightly bonded together and so the entire ion behaves as a single unit. One Cl atom can only pick one electron therefore, the two electrons are accepted by 2 Cl atoms to give 2Cl - anions. The formula of the carbonate ion is CO 32. It is composed of Ca 2+ cations and Cl - anions those ions are stable since they have filled valence shells. The Group 2 Calcium (Ca) atom can easily lose two electrons, therefore, The element B from the Group 7 and period 3 is Chlorine (Cl). The principal quantum number of the outermost valence shell of element B is 3 therefore, the element belongs to the 3 rd period (row) of the periodic table. Therefore, halogens have high negative electron gain enthalpy values and attain electrons easily. The halogens are less by one electron from attaining the stable inert gas configuration. The principal quantum number of the outermost shell of element A is 4 therefore, the element belongs to the 4 th period (row) of the periodic table.Įlement A belonging to Group 2 and period 4, is Calcium (Ca).ī belongs to group 7 based on the valence shell electronic configuration of 7. Group 2 elements can therefore lose two electrons to attain stability. Metals have low ionization enthalpy they can easily lose electrons to attain the nearest stable, inert gas configuration. Therefore, A belongs to the Group 2 of the periodic table.
What is the formula of the ionic compound formed between these elements?Ī has a valence shell configuration of 2. Ionic Compound Formula Sodium chloride: NaCl, with Na+ and Cl ions Lithium nitride: Li3N, with Li+ and N3- ions Magnesium oxide: MgO, with Mg2+ and O2- ions. The electronic configuration of two elements A and B are.


 0 kommentar(er)
0 kommentar(er)
